
Bromothymol blue is a pH indicator that switches between different colors depending on the pH of the solution. It occurs in protonated (yellow) and deprotonated (blue) forms, with a greenish color in neutral solutions. This color switch is due to the deprotonation activity, which improves the molecule’s structure and absorption properties. It is synthesized by adding elemental bromine to thymol blue in glacial acetic acid.
CAS No.: 76-59-5
Synonyms: Dibromothymolsulfophthalein; 3,3′-Dibromothymolsulfonphthalein; Bromthymolblau; Azul de bromotimol; Blu di bromotimolo; Bleu de bromothymol
| Physical Properties | |
| Chemical formula | C27H28Br2O5S |
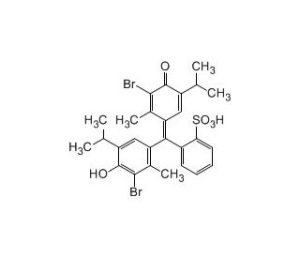
| IUPAC Name | 2-bromo-4-[3-(3-bromo-4-hydroxy-2-methyl-5-propan-2-ylphenyl)-1,1-dioxo-2,1λ6-benzoxathiol-3-yl]-3-methyl-6-propan-2-ylphenol |
| Molecular weight | 624.4g/mol |
| Solubility | Water, ether, and alkalis less soluble in benzene and toluene |
| Insoluble | Petroleum ether |
| Flash point | 38°C |
| Density | 1.25 g/cm3 |
| pKa | 7.0 |
| Chemical Properties | |
| Colour | White to cream |
| State | Solid powder |
| Melting point | 200-202°C |
| λmax | 420 nm |
| Pictograms : |  |
| Hazard Statements : | H226: Flammable liquid and vapour. |
| Precautionary statements : | P210: Keep away from heat/sparks/open flames/hot surfaces. – No smoking. |
Bromothymol blue is a pH indicator that switches between different colors depending on the pH of the solution. It occurs in protonated (yellow) and deprotonated (blue) forms, with a greenish color in neutral solutions. This color switch is due to the deprotonation activity, which improves the molecule’s structure and absorption properties. It is synthesized by adding elemental bromine to thymol blue in glacial acetic acid.

CAS No.: 76-59-5
Synonyms: Dibromothymolsulfophthalein; 3,3′-Dibromothymolsulfonphthalein; Bromthymolblau; Azul de bromotimol; Blu di bromotimolo; Bleu de bromothymol
| Physical Properties | |
| Chemical formula | C27H28Br2O5S |
| IUPAC Name | 2-bromo-4-[3-(3-bromo-4-hydroxy-2-methyl-5-propan-2-ylphenyl)-1,1-dioxo-2,1λ6-benzoxathiol-3-yl]-3-methyl-6-propan-2-ylphenol |
| Molecular weight | 624.4g/mol |
| Solubility | Water, ether, and alkalis less soluble in benzene and toluene |
| Insoluble | Petroleum ether |
| Flash point | 38°C |
| Density | 1.25 g/cm3 |
| pKa | 7.0 |
| Chemical Properties | |
| Colour | White to cream |
| State | Solid powder |
| Melting point | 200-202°C |
| λmax | 420 nm |
| Pictograms : |  |
| Hazard Statements : | H226: Flammable liquid and vapour. |
| Precautionary statements : | P210: Keep away from heat/sparks/open flames/hot surfaces. – No smoking. |
Bromothymol blue is a pH indicator that changes color with acidity or alkalinity. It turns yellow in acidic solutions, green in neutral solutions, and blue in basic solutions.
Bromothymol blue is a pH indicator that changes color due to its molecular structure’s interaction with hydrogen ions (H+). In acidic solutions, the molecule’s structure changes, causing it to absorb blue light and transmit yellow light, making the solution appear yellow. In neutral and basic solutions, the molecule’s structure is different, leading to the absorption of red light and the transmission of blue light, resulting in a blue color.
Bromothymol blue is a pH indicator used in titrations to signal the endpoint of neutralization reactions. Its distinct color change (yellow to blue or vice versa) makes it useful for various applications, such as checking the pH of swimming pool water.
Bromothymol blue is a versatile pH indicator used in various applications. It’s employed to measure the pH of solutions, monitor biological processes like photosynthesis and cellular respiration, test water quality in swimming pools and aquariums, and even detect premature membrane rupture in obstetrics.