
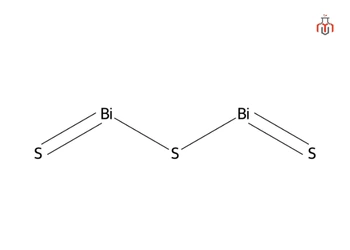
Bismuth sulfide is a chemical compound consisting of bismuth and sulfide and occurs in nature as bismuthinite. This compound can be synthesized by reacting bismuth(III) salts with hydrogen sulfide or heating elemental bismuth and sulfur together. Bismuth sulfide has a structure similar to stibnite (antimony(III) sulfide), with bismuth atoms arranged in distinct coordination environments. It can react with acids to release hydrogen sulfide gas and may form in the body under certain conditions, leading to temporary discoloration of the tongue and feces.
CAS No.: 1345-07-9
Synonyms: sulfanylidene(sulfanylidenebismuthanylsulfanyl)bismuthane; Bismuth(III) sulfide, 99%; Bismuth(iii)sulfide; Wismutsulfid; Bismuth sulphide; EINECS 215-716-0; UNII-XZC47M60X8; BAA34507; SY061334; Tantalum Silicide (TaSi2) Sputtering Targets.
| Physical Properties | |
| Chemical formula | Bi2S3 |
| IUPAC Name | sulfanylidene(sulfanylidenebismuthanylsulfanyl)bismuthane |
| Molecular weight | 514.2 g/mol |
| Density | 6.78 g/cm³ |
| Chemical Properties | |
| Color | Brown – Black |
| State | Powder |
| Melting point | 850 °C |
| Magnetic susceptibility | -123.0·10−6 cm3/mol |
| Pictograms : |  |
| Hazard Statements : | H315: Causes skin irritation. |
| Precautionary statements : | P261: Avoid breathing dust/ fume/ gas/ mist/ vapors/ spray. P264: Wash skin thoroughly after handling. P271: Use only outdoors or in a well-ventilated area. P280: Wear protective gloves/ eye protection/ face protection. P302 + P352: IF ON SKIN: Wash with plenty of water. P305 + P351 + P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present, and easy to do. Continue rinsing. |
Bismuth sulfide is a chemical compound consisting of bismuth and sulfide and occurs in nature as bismuthinite. This compound can be synthesized by reacting bismuth(III) salts with hydrogen sulfide or heating elemental bismuth and sulfur together. Bismuth sulfide has a structure similar to stibnite (antimony(III) sulfide), with bismuth atoms arranged in distinct coordination environments. It can react with acids to release hydrogen sulfide gas and may form in the body under certain conditions, leading to temporary discoloration of the tongue and feces.

CAS No.: 1345-07-9
Synonyms: sulfanylidene(sulfanylidenebismuthanylsulfanyl)bismuthane; Bismuth(III) sulfide, 99%; Bismuth(iii)sulfide; Wismutsulfid; Bismuth sulphide; EINECS 215-716-0; UNII-XZC47M60X8; BAA34507; SY061334; Tantalum Silicide (TaSi2) Sputtering Targets.
| Physical Properties | |
| Chemical formula | Bi2S3 |
| IUPAC Name | sulfanylidene(sulfanylidenebismuthanylsulfanyl)bismuthane |
| Molecular weight | 514.2 g/mol |
| Density | 6.78 g/cm³ |
| Chemical Properties | |
| Color | Brown – Black |
| State | Powder |
| Melting point | 850 °C |
| Magnetic susceptibility | -123.0·10−6 cm3/mol |
| Pictograms : |  |
| Hazard Statements : | H315: Causes skin irritation. |
| Precautionary statements : | P261: Avoid breathing dust/ fume/ gas/ mist/ vapors/ spray. P264: Wash skin thoroughly after handling. P271: Use only outdoors or in a well-ventilated area. P280: Wear protective gloves/ eye protection/ face protection. P302 + P352: IF ON SKIN: Wash with plenty of water. P305 + P351 + P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present, and easy to do. Continue rinsing. |
Bismuth sulfide is used as a starting material for producing other bismuth compounds and in various applications in electronics, thermoelectrics, and semiconductors.
Bismuth sulfide (Bi₂S₃) is insoluble in sodium hydroxide (NaOH). It does not dissolve in alkaline solutions, as it is generally insoluble in acids and bases.
Bismuth sulfide is generally considered low in toxicity, but its safety depends on the exposure level. It should be handled with care, avoiding inhalation or ingestion.
Bismuth sulfide is formed by reacting bismuth(III) salts with hydrogen sulfide or by heating elemental bismuth and sulfur together in an evacuated tube at high temperatures.
The ore of Bi₂S₃ (Bismuth(III) sulfide) is bismuthinite, a naturally occurring mineral composed mainly of bismuth and sulfur.