

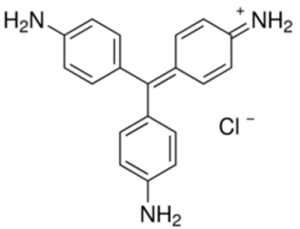
Basic Fuchsin, also known as Fuchsine or Rosaniline hydrochloride, in its solid form, appears as dark green crystals that turn magenta when dissolved in water. The dye consists of a mixture of compounds, including rosaniline, pararosaniline, new fuchsine, and Magenta II. Its structure features three amine groups, which contribute to its basic properties, allowing it to form salts.
CAS No.: 632-99-5
Synonyms: Rosaniline hydrochloride; Basic violet 14; Fuchsin basic; Fuchsin; Rosaniline; Basic Magenta; Diamond Fuchsin; Fuchsine
| Physical Properties | |
| Chemical formula | C20H20ClN3 |
| IUPAC Name | 4-[(4-aminophenyl)-(4-imino-3-methylcyclohexa-2,5-dien-1-ylidene)methyl]aniline;hydrochloride |
| Molecular weight | 337.8 g/mol |
| Solubility | Soluble in Water, Alcohol and Diethyl ether |
| Density | 0.999 g/mL at 20 °C |
| Chemical Properties | |
| Colour | Dark Green |
| State | Crystalline Solid powder |
| Melting point | 200°C |
| λmax | 543 nm |
| Log P | 2.920 |
| Vapor pressure | 7.49×10−10 mmHg (25 °C) |
Biological Staining: Basic Fuchsine is employed as a stain in biological studies, particularly for staining cell nuclei, bacteria, and other cellular components.
Acid-Fast Staining: Basic fuchsine is a vital element in the Ziehl-Neelsen stain, as it helps in identifying acid-fast bacteria like Mycobacterium tuberculosis.
Schiff Reagent: Basic Fuchsine is used in preparing the Schiff reagent, which helps in detecting aldehydes, commonly in the periodic acid-Schiff (PAS) reaction for staining carbohydrates.
Mounting Media: Basic Fuchsin is also a constituent of Lactofuchsin, a mounting medium for preserving stained specimens.
| Pictograms : |   |
| Hazard Statements : | H350: May cause cancer |
| Precautionary statements : | P201: Obtain special instructions before use. |
Basic Fuchsin, also known as Fuchsine or Rosaniline hydrochloride, in its solid form, appears as dark green crystals that turn magenta when dissolved in water. The dye consists of a mixture of compounds, including rosaniline, pararosaniline, new fuchsine, and Magenta II. Its structure features three amine groups, which contribute to its basic properties, allowing it to form salts.

CAS No.: 632-99-5
Synonyms: Rosaniline hydrochloride; Basic violet 14; Fuchsin basic; Fuchsin; Rosaniline; Basic Magenta; Diamond Fuchsin; Fuchsine
| Physical Properties | |
| Chemical formula | C20H20ClN3 |
| IUPAC Name | 4-[(4-aminophenyl)-(4-imino-3-methylcyclohexa-2,5-dien-1-ylidene)methyl]aniline;hydrochloride |
| Molecular weight | 337.8 g/mol |
| Solubility | Soluble in Water, Alcohol and Diethyl ether |
| Density | 0.999 g/mL at 20 °C |
| Chemical Properties | |
| Colour | Dark Green |
| State | Crystalline Solid powder |
| Melting point | 200°C |
| λmax | 543 nm |
| Log P | 2.920 |
| Vapor pressure | 7.49×10−10 mmHg (25 °C) |
Biological Staining: Basic Fuchsine is employed as a stain in biological studies, particularly for staining cell nuclei, bacteria, and other cellular components.
Acid-Fast Staining: Basic fuchsine is a vital element in the Ziehl-Neelsen stain, as it helps in identifying acid-fast bacteria like Mycobacterium tuberculosis.
Schiff Reagent: Basic Fuchsine is used in preparing the Schiff reagent, which helps in detecting aldehydes, commonly in the periodic acid-Schiff (PAS) reaction for staining carbohydrates.
Mounting Media: Basic Fuchsin is also a constituent of Lactofuchsin, a mounting medium for preserving stained specimens.
| Pictograms : |   |
| Hazard Statements : | H350: May cause cancer |
| Precautionary statements : | P201: Obtain special instructions before use. |
Basic Fuchsin is polar. It contains multiple amine groups which can form hydrogen bonds, contributing to its overall polarity.
A Basic Fuchsin stain is a magenta-colored dye used in microscopy to stain bacteria, cell nuclei, and other cellular components.
Basic fuchsin dye binds to acidic components of cells, highlighting structures like bacteria, nuclei, and connective tissues in microscopy.
Basic fuchsin stain is used to identify bacteria by staining them red or magenta, making them visible under a microscope, especially in procedures like acid-fast staining.