

Giemsa is a Romanowsky-type stain mixture of Methylene blue, Azure B dyes (an oxidized form of methylene blue), Eosin Y, Glycerol, and Methanol. It was discovered by German chemist and bacteriologist Gustav Giemsa in the year 1902. It is the most widely used stain in hematology, histology, bacteriology, and cytology.
It helps in detecting blood-borne parasites like malaria, identifying bacterial infections such as Chlamydia, H. pylori, and gonorrhea, and spotting fungal elements in tissue samples. In hematology, it enhances blood smears, helping to differentiate lymphocytes and other cell types.
| Ingredients | gm/L |
| Giemsa Powder | 7.6g/L (methylen blue, eosin Y, and azure dyes) |
| Glycerol | 500 ml/L |
| Methanol | 500 ml/L |
CAS No.: 51811-82-6
Synonyms: Mixture of Azure II Eosinate & Methylene Blue; mancha de giemsa; tinción de giemsa; giemsa labe; tache de giemsa
Resources: Biological Stains | Classification, Examples & Uses
| Physical Properties | |
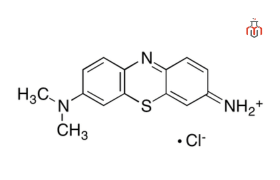
| Chemical formula | C14H14ClN3S |
| IUPAC Name | 7-imino-N,N-dimethylphenothiazin-3-amine;hydrochloride |
| Molecular weight | 291.8 g/mol |
| Solubility | Soluble in Water & Ethanol |
| Flash point | 12°C |
| Density | 1.04 g/cm3 |
| Chemical Properties | |
| Colour | Dark Green to Black |
| State | Crystal or powder |
| Melting point | 300°C |
| Vapour Pressure | 97.68mm at 20°C |
| pH | 7 |
| Vapour density | 2.37 |
| Loss on Drying | NMT 10% (110°C) |
Giemsa stain is a Romanowsky stain that binds to acidic and basic cell components. Azure B and methylene blue stain nuclei and ribosomal RNA blue to purple, while eosin stains cytoplasm pink to red. This staining technique helps differentiate blood cells and detect parasites like Plasmodium (malaria).
| Pictograms : |    |
| Hazard Statements : | H225: Highly flammable liquid and vapour H301+H311: Toxic if swallowed or in contact with skin H370: Causes damage to organs H318: Causes serious eye damage |
| Precautionary statements : | P301+P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P210: Keep away from heat, hot surfaces, sparks, open flames, and other ignition sources. No smoking. P280: Wear protective gloves/protective clothing/eye protection/face protection. P304+P312: IF INHALED: Call a POISON CENTER or doctor/physician if you feel unwell. P352: Wash with plenty of water. |
Giemsa is a Romanowsky-type stain mixture of Methylene blue, Azure B dyes (an oxidized form of methylene blue), Eosin Y, Glycerol, and Methanol. It was discovered by German chemist and bacteriologist Gustav Giemsa in the year 1902. It is the most widely used stain in hematology, histology, bacteriology, and cytology.
It helps in detecting blood-borne parasites like malaria, identifying bacterial infections such as Chlamydia, H. pylori, and gonorrhea, and spotting fungal elements in tissue samples. In hematology, it enhances blood smears, helping to differentiate lymphocytes and other cell types.

| Ingredients | gm/L |
| Giemsa Powder | 7.6g/L (methylen blue, eosin Y, and azure dyes) |
| Glycerol | 500 ml/L |
| Methanol | 500 ml/L |
CAS No.: 51811-82-6
Synonyms: Mixture of Azure II Eosinate & Methylene Blue; mancha de giemsa; tinción de giemsa; giemsa labe; tache de giemsa
Resources: Biological Stains | Classification, Examples & Uses
| Physical Properties | |
| Chemical formula | C14H14ClN3S |
| IUPAC Name | 7-imino-N,N-dimethylphenothiazin-3-amine;hydrochloride |
| Molecular weight | 291.8 g/mol |
| Solubility | Soluble in Water & Ethanol |
| Flash point | 12°C |
| Density | 1.04 g/cm3 |
| Chemical Properties | |
| Colour | Dark Green to Black |
| State | Crystal or powder |
| Melting point | 300°C |
| Vapour Pressure | 97.68mm at 20°C |
| pH | 7 |
| Vapour density | 2.37 |
| Loss on Drying | NMT 10% (110°C) |
Giemsa stain is a Romanowsky stain that binds to acidic and basic cell components. Azure B and methylene blue stain nuclei and ribosomal RNA blue to purple, while eosin stains cytoplasm pink to red. This staining technique helps differentiate blood cells and detect parasites like Plasmodium (malaria).
| Pictograms : |    |
| Hazard Statements : | H225: Highly flammable liquid and vapour H301+H311: Toxic if swallowed or in contact with skin H370: Causes damage to organs H318: Causes serious eye damage |
| Precautionary statements : | P301+P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P210: Keep away from heat, hot surfaces, sparks, open flames, and other ignition sources. No smoking. P280: Wear protective gloves/protective clothing/eye protection/face protection. P304+P312: IF INHALED: Call a POISON CENTER or doctor/physician if you feel unwell. P352: Wash with plenty of water. |
Giemsa stain is widely regarded as the world’s standard diagnostic technique for identifying Plasmodium, the parasite that causes malaria. It also serves as the basic stain for classifying lymphomas according to the Kiel classification.
Both Giemsa stain and Giemsa-Wright stain contain Methylene blue, Azure B, and eosin Y, but they differ in formulation, solvent, staining properties, and applications. Giemsa stain is a single stain used for staining blood parasites like malaria and cytoplasm of cells pink to red, and the nuclei blue to purple, while Wright-Giemsa is a two-step staining method that combines Wright stain (for differentiating blood cell types) with Giemsa stain to enhance nuclear staining, peripheral blood smears and bone marrow specimens.
Giemsa stain is used to detect Chlamydia trachomatis in infected cells by highlighting intracytoplasmic inclusions. However, molecular techniques are now preferred for higher accuracy.
Giemsa stain contains methanol, which is flammable and toxic if inhaled or absorbed.
Giemsa stain is a differential stain composed of both basic and acidic dyes.
Giemsa stain is a differential stain. It is composed of eosin and azure, both of which are acidic dyes, and methylene blue, which is a basic dye.
Giemsa stain is used to detect Helicobacter pylori, Borrelia, Plasmodium (malaria parasite), Trypanosoma, and Chlamydia species, etc.
It stains the phosphate groups of DNA, making it ideal for visualizing chromosomes and chromatin. It can also stain the cytoplasm and microorganisms like malaria parasites, Chlamydia, and Borrelia.
Giemsa stain is composed of methylene blue, eosin, and azure dyes dissolved in a methanol-glycerol solution. It is commonly used for staining blood smears and microbial specimens.
Field stain is a rapid staining method primarily used in field diagnostics, while Giemsa stain provides a more detailed visualization of cell morphology but requires a longer processing time.