

Malachite Green is an organic compound, classified as a triarylmethane dye. It has an intense green color. The dye is not extracted from the mineral malachite, instead, its name is inspired by the color similarity. The compound is generally found in its chloride form, though other salts like oxalate are also present. The dye’s intense color occurs through the cationic form, which enables enlarged pi-delocalization and permits visible light absorption. Moreover, it is synthesized through the condensation of benzaldehyde and dimethylaniline, followed by oxidation. Its structural composition allows it to traverse cell membranes, where it is metabolized into a leuco form. This form is not colored, differentiating it from the highly colored cationic version.
CAS No.: 4569-64-2 , 2437-29-8
Synonyms: Aniline green; Benzaldehyde green; China green; Acryl Brilliant Green; Fast Green; Victoria Green; Basic Green 4; Diamond green B; C.I 42000
Resources: Biological Stains | Classification, Examples & Uses
| Physical Properties | |
| Chemical formula | C₂₃H₂₅ClN₂ |
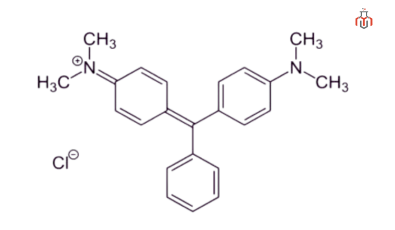
| IUPAC name | 4-{[4-(Dimethylamino)phenyl](phenyl)methylidene}-N,N-dimethylcyclohexa-2,5-dien-1-iminium chloride |
| Molecular weight | 364.9 g/mol |
| Solubility | Soluble in water, ethanol, methanol, and amyl alcohol, Slightly soluble in chloroform and benzene |
| Flash point | 45°C |
| Density | 1.03 g/cm3 at 20°C |
| Chemical Properties | |
| Colour | Dark Green |
| State | Crystal or powder |
| Melting point | 158-160ºC |
| Odor | Odorless |
| pH Sensitivity | Green in neutral/alkaline, yellow in acidic |
| Pictograms : |
|
| Hazard Statements : | H226: Flammable liquid and vapor H315: Causes skin irritation H319: Causes serious eye irritation H412: Harmful to aquatic life with long-lasting effects |
| Precautionary statements : | P210: Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. P233: Keep the container tightly closed. |
Malachite Green is an organic compound, classified as a triarylmethane dye. It has an intense green color. The dye is not extracted from the mineral malachite, instead, its name is inspired by the color similarity. The compound is generally found in its chloride form, though other salts like oxalate are also present. The dye’s intense color occurs through the cationic form, which enables enlarged pi-delocalization and permits visible light absorption. Moreover, it is synthesized through the condensation of benzaldehyde and dimethylaniline, followed by oxidation. Its structural composition allows it to traverse cell membranes, where it is metabolized into a leuco form. This form is not colored, differentiating it from the highly colored cationic version.

CAS No.: 4569-64-2 , 2437-29-8
Synonyms: Aniline green; Benzaldehyde green; China green; Acryl Brilliant Green; Fast Green; Victoria Green; Basic Green 4; Diamond green B; C.I 42000
Resources: Biological Stains | Classification, Examples & Uses
| Physical Properties | |
| Chemical formula | C₂₃H₂₅ClN₂ |
| IUPAC name | 4-{[4-(Dimethylamino)phenyl](phenyl)methylidene}-N,N-dimethylcyclohexa-2,5-dien-1-iminium chloride |
| Molecular weight | 364.9 g/mol |
| Solubility | Soluble in water, ethanol, methanol, and amyl alcohol, Slightly soluble in chloroform and benzene |
| Flash point | 45°C |
| Density | 1.03 g/cm3 at 20°C |
| Chemical Properties | |
| Colour | Dark Green |
| State | Crystal or powder |
| Melting point | 158-160ºC |
| Odor | Odorless |
| pH Sensitivity | Green in neutral/alkaline, yellow in acidic |
| Pictograms : |
|
| Hazard Statements : | H226: Flammable liquid and vapor H315: Causes skin irritation H319: Causes serious eye irritation H412: Harmful to aquatic life with long-lasting effects |
| Precautionary statements : | P210: Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. P233: Keep the container tightly closed. |
Malachite Green enters an endospore through heat application, which allows the dye to penetrate the tough outer layer. Once inside, it binds to the endospore, staining it green while other cell parts remain unstained or take up a counterstain.
Malachite Green is primarily used as a dye in textiles and as a biological stain in microscopy. It’s also used in aquaculture to treat infections and in forensic science to detect latent blood.
Malachite Green is an antifungal. It is used to treat fungal infections in aquaculture, particularly against the oomycete Saprolegnia.
Malachite green may damage the DNA as it can produce genotoxic, carcinogenic, and mutagenic effects on living beings.
Yes, Malachite Green can kill both harmful and beneficial bacteria. It is not selective and can affect a wide range of microorganisms, including those beneficial to the ecosystem.
If Malachite Green gets wet, it can dissolve or become more concentrated in the water, potentially causing staining. The dye can spread and leave green residues on surfaces or materials.
Malachite green is toxic to humans and is not safe for medical use. It can cause serious eye problems and respiratory toxicity. It can also lead to damage to the DNA.
Even low-concentration Malachite green is not considered safe to use in turtles as it has soft tissue that absorbs harmful chemicals.