
Methyl orange, a pH indicator is often used in titration as it exhibits a definite color change over a particular pH range. It appears red in the acidic medium and yellow in the basic medium, shifting from red to orange and then yellow as the solution becomes less acidic. Methyl orange has a pKa of 3.47 in water at 25 °C, exhibiting its sensitivity to pH changes in the range of 3.1 to 4.4. It is an azobenzene derivative, synthesized from dimethylaniline and sulfanilic acid through diazonium salt formation and nucleophilic attack. Its UV-vis absorption spectrum peaks at 464 nm, aiding its usual orange color.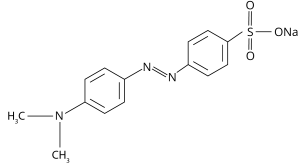
CAS No.: 547-58-0
Synonyms: Orange III; MethylOrange; Gold Orange; Helianthine B; C.I. Acid Orange 52; Eniamethyl Orange; Tropaeolin D; Sodium p-dimethylaminoazobenzenesulfonate
| Physical Properties | |
| Chemical formula | C14H14N3NaO3S |
| IUPAC Name | Sodium 4-{[4-(dimethylamino)phenyl]diazenyl}benzene-1-sulfonate |
| Molecular weight | 327.33 g/mol |
| Solubility | Soluble in Water and Ethanol |
| Flash Point | 248°C |
| Density | 1.28 g/cm³ |
| Chemical Properties | |
| Colour | Orange-yellow |
| State | Crystalline Solid Powder |
| Melting point | 300°C |
| pKa | 3.47 at 25°C |
| Pictograms : |
|
| Hazard Statements : | H301: Toxic if swallowed. |
| Precautionary statements : | P264: Wash skin thoroughly after handling. |
Methyl orange, a pH indicator is often used in titration as it exhibits a definite color change over a particular pH range. It appears red in the acidic medium and yellow in the basic medium, shifting from red to orange and then yellow as the solution becomes less acidic. Methyl orange has a pKa of 3.47 in water at 25 °C, exhibiting its sensitivity to pH changes in the range of 3.1 to 4.4. It is an azobenzene derivative, synthesized from dimethylaniline and sulfanilic acid through diazonium salt formation and nucleophilic attack. Its UV-vis absorption spectrum peaks at 464 nm, aiding its usual orange color.
CAS No.: 547-58-0
Synonyms: Orange III; MethylOrange; Gold Orange; Helianthine B; C.I. Acid Orange 52; Eniamethyl Orange; Tropaeolin D; Sodium p-dimethylaminoazobenzenesulfonate
| Physical Properties | |
| Chemical formula | C14H14N3NaO3S |
| IUPAC Name | Sodium 4-{[4-(dimethylamino)phenyl]diazenyl}benzene-1-sulfonate |
| Molecular weight | 327.33 g/mol |
| Solubility | Soluble in Water and Ethanol |
| Flash Point | 248°C |
| Density | 1.28 g/cm³ |
| Chemical Properties | |
| Colour | Orange-yellow |
| State | Crystalline Solid Powder |
| Melting point | 300°C |
| pKa | 3.47 at 25°C |
| Pictograms : |
|
| Hazard Statements : | H301: Toxic if swallowed. |
| Precautionary statements : | P264: Wash skin thoroughly after handling. |
It changes color between pH 3.1 and 4.4, shifting from red in acidic conditions to yellow in basic conditions.
Methyl orange is polar due to the presence of sulfonic acid and azo groups, which create regions of partial positive and negative charges, leading to its solubility in polar solvents like water.
The pH range of methyl orange is from 3.1 to 4.4.
Methyl orange is much more suitable for titrations with strong acids and weak bases due to its clear color change in the pH range of 3.1 to 4.4, providing a sharp endpoint. On the contrary, phenolphthalein is more appropriate for titrations with strong bases and weak acids.
In neutral solutions, methyl orange is orange.