
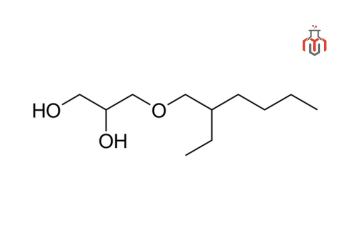
Ethylhexylglycerin (C₁₁H₂₄O₃), also known as octoxyglycerin, is a glyceryl ether. Chemically, it is a derivative of glycerin, where one of the hydrogen atoms in the glycerin molecule is replaced by an ethylhexyl group. It is a synthetic compound derived from plant sources of glycerin, such as coconut, palm and soy. It was introduced in 1990 as a preservative alternative by a german scientist, but it quickly gained popularity for its hydration, deodorization, and product stabilization properties. Today, it is a widely used ingredient in personal care products, valued for its mild, biodegradable nature and compatibility with sensitive skin.
CAS No.: 70445-33-9
Synonyms: Ethyl hexyl glycerin; Octoxyglycerin; Etilhexilglicerina; Éthylhexylglycérine
| Physical Properties | |
| Chemical formula | C11H24O3 |
| IUPAC Name | 3-ethylnonane-1,2,3-triol |
| Molecular weight | 204.31 g/mol |
| Solubility | Slightly soluble in water |
| Density | 0.954 g/ml |
| Chemical Properties | |
| Color | Colorless |
| State | Liquid |
| Boiling point | 325°C |
| Moisture Content | NMT 0.2% |
| Refractive Index | 1.450-1.455 |
| Pictograms : | 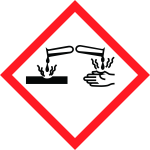 |
| Hazard Statements : | H318: Causes serious eye damage H412: Harmful to aquatic life with long-lasting effects |
| Precautionary statements : | P280: Wear protective gloves/protective clothing/eye protection/face protection/hearing protection P305+P354+P338 IF IN EYES: Immediately rinse with water for several minutes. Remove contact lenses, if present. Continue rinsing |
Ethylhexylglycerin (C₁₁H₂₄O₃), also known as octoxyglycerin, is a glyceryl ether. Chemically, it is a derivative of glycerin, where one of the hydrogen atoms in the glycerin molecule is replaced by an ethylhexyl group. It is a synthetic compound derived from plant sources of glycerin, such as coconut, palm and soy. It was introduced in 1990 as a preservative alternative by a german scientist, but it quickly gained popularity for its hydration, deodorization, and product stabilization properties. Today, it is a widely used ingredient in personal care products, valued for its mild, biodegradable nature and compatibility with sensitive skin.

CAS No.: 70445-33-9
Synonyms: Ethyl hexyl glycerin; Octoxyglycerin; Etilhexilglicerina; Éthylhexylglycérine
| Physical Properties | |
| Chemical formula | C11H24O3 |
| IUPAC Name | 3-ethylnonane-1,2,3-triol |
| Molecular weight | 204.31 g/mol |
| Solubility | Slightly soluble in water |
| Density | 0.954 g/ml |
| Chemical Properties | |
| Color | Colorless |
| State | Liquid |
| Boiling point | 325°C |
| Moisture Content | NMT 0.2% |
| Refractive Index | 1.450-1.455 |
| Pictograms : |  |
| Hazard Statements : | H318: Causes serious eye damage H412: Harmful to aquatic life with long-lasting effects |
| Precautionary statements : | P280: Wear protective gloves/protective clothing/eye protection/face protection/hearing protection P305+P354+P338 IF IN EYES: Immediately rinse with water for several minutes. Remove contact lenses, if present. Continue rinsing |
Ethylhexylglycerin is safe to use at low doses. It may cause skin irritation if it is used in rinse-off products at concentrations greater than 8% and in leave-on products at concentrations greater than 2%.
Ethylhexylglycerin does not clog pores and is not comedogenic.
There is no scientific evidence to suggest that ethylhexylglycerin is carcinogenic. It has been assessed for safety and is widely used in cosmetics.
Yes, ethylhexylglycerin is primarily derived from plant-based sources such as palm or coconut oil.
Studies have not indicated any reproductive toxicity associated with ethylhexylglycerin.
Ethylhexylglycerin is used as a substitute for parabens and is a biodegradable preservative.
Ethylhexylglycerin has the ability to prevent hair from becoming dry & brittle, reduces frizz, and cleanses the scalp. It also makes hair shinier and smoother.
Ethylhexylglycerin is considered safe for use in cosmetics and skincare products. It has a low risk of toxicity and is generally well-tolerated by the skin.