
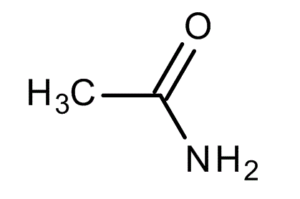
Acetamide (ethanamide) is an organic compound having the molecular formula CH3CONH2, synthesised from ammonia and acetic acid. It undergoes acidic or basic hydrolysis to form acetic acid and ammonia or ammonium ion, and can react with strong acids or bases due to the presence of the amide linkage. It is commonly used as a plasticizer and as a solvent in an industrial scale.
CAS No.: 60-35-5
Synonyms: Ethanamide; Acetic acid amide; Methanecarboxamide; Acetimidic acid; Ethanimidic acid.
| Physical Properties | |
| Chemical formula | CH3CONH2 |
| IUPAC Name | acetamide |
| Molecular weight | 59.07 g/mol |
| Solubility | Water & Ethanol |
| Density | 1.585 g/cm³ |
| Flash point | 126 °C |
| Chemical Properties | |
| Color | Colorless |
| State | Solid Crystals |
| Melting point | 79 – 81 °C |
| Log P | −1.26 |
| pKa | 15.1 (at 25 °C) |
| Pictograms : |  |
| Hazard Statements : | H351: Suspected of causing cancer. |
| Precautionary statements : | P201: Obtain special instructions before use. P202: Do not handle until all safety precautions have been read and understood. P280: Wear protective gloves/ protective clothing/ eye protection/ face protection. P308 + P313: IF exposed or concerned: Get medical advice/ attention. P405: Store locked up. P501: Dispose of contents/ container to an approved waste disposal plant. |
Acetamide (ethanamide) is an organic compound having the molecular formula CH3CONH2, synthesised from ammonia and acetic acid. It undergoes acidic or basic hydrolysis to form acetic acid and ammonia or ammonium ion, and can react with strong acids or bases due to the presence of the amide linkage. It is commonly used as a plasticizer and as a solvent in an industrial scale.

CAS No.: 60-35-5
Synonyms: Ethanamide; Acetic acid amide; Methanecarboxamide; Acetimidic acid; Ethanimidic acid.
| Physical Properties | |
| Chemical formula | CH3CONH2 |
| IUPAC Name | acetamide |
| Molecular weight | 59.07 g/mol |
| Solubility | Water & Ethanol |
| Density | 1.585 g/cm³ |
| Flash point | 126 °C |
| Chemical Properties | |
| Color | Colorless |
| State | Solid Crystals |
| Melting point | 79 – 81 °C |
| Log P | −1.26 |
| pKa | 15.1 (at 25 °C) |
| Pictograms : |  |
| Hazard Statements : | H351: Suspected of causing cancer. |
| Precautionary statements : | P201: Obtain special instructions before use. P202: Do not handle until all safety precautions have been read and understood. P280: Wear protective gloves/ protective clothing/ eye protection/ face protection. P308 + P313: IF exposed or concerned: Get medical advice/ attention. P405: Store locked up. P501: Dispose of contents/ container to an approved waste disposal plant. |
Yes, acetamide can form hydrogen bonds due to the presence of both a carbonyl group (C=O) and an amide group (NH2).
Acetamide is polar due to its amide group, which consists of a polar carbonyl (C=O) and amine (NH2) group, leading to an uneven dispersal of charges.
Acetamide is employed as a solvent in various industrial processes, a plasticizer for polymers, and an intermediate in the synthesis of pharmaceuticals, pesticides, and other organic compounds.
Acetamide is usually considered neutral, but it can exhibit weak acidic behavior due to the presence of the amide group (–CONH2). However, it is not strongly acidic and does not behave like typical acids.
Acetamide usually serves as a solvent and a chemical intermediate. It assists in organic synthesis and electrochemical processes due to its high dielectric constant.